Marking contrast can be achieved by surface material removal or color change. When infrared lasers are used, marking contrast relies on thermal effects. When UV lasers, such as excimer lasers are used, marking contrast can be achieved through a photo-chemical transformation, i.e., color change. The process is non-thermal and thus has its unique markets for applications.
1. Laser Marking Mechanism
When a laser beam is focused on the surface of a target material, many phenomena may occur as shown in Figure 1.
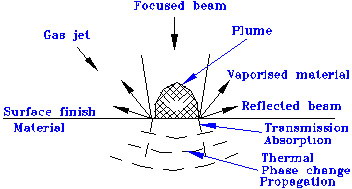
Figure 1: Physical phenomena in laser marking
(1) Vaporization
The laser beam is focused to a small spot, which greatly increases the energy density. When the energy is high enough, and the surface temperature is raised well above the melting point, most of the material on which the beam is focused will vaporize. The efficiency of this vaporization process depends on the absorption of the wavelength of the laser radiation. Organic materials and certain glasses have very good absorption of the 10.6 mm wavelength, often 100%. Metals absorb the 1.06 mm wavelength very well but a fraction of the laser energy will be reflected, which may be dangerous.
In the marking process, the energy density used is often high enough that the desired vaporization completes in microseconds. A series of vaporized craters in a surface usually alters its appearance sufficiently to be visible if characters are formed. The marking contrast depends on the chemistry of the material, the surface finish and color. A good mark edge resolution is achievable. The mark depth and width is controllable. Materials such as plastic, glass, ceramic, rubber and metals will be slightly engraved with a distinct change of the surface structure.
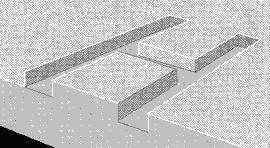
Figure 2: Marking through material removal
(2) Softening/Melting
Some materials are melted by infrared laser radiation, e.g. metals, epoxies and glass (Figure 3). In the case of metals, mark contrast is achieved by oxidation or incorporation of impurities into the melt. In the case of plastics, the material melts and forms ridges. Depending on types of material, different colors may appear. If the energy density exceeds the ignition point, carbonization occurs, which leads to black lettering. The durability is, however, not good since the carbonized material will wear off, impairing the legibility.
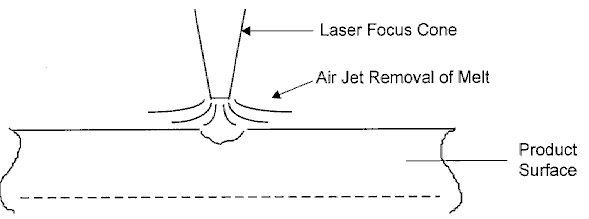
Figure 3: Softening and melting marking
(3) Layer removal/ablation
Layer removal/ablation is actually a form of controlled vaporisation (Figure 4). A thin layer of plastic film, paper, ink or paint is vaporized exposing the different colored under-layer. By controlling the heat input, the depth material removed can be controlled and the damage to the underlying surface minimized. Applications of this type of marking include instrumentation panels and consumer products etc..
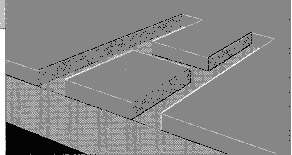
Marking through layer removal
(4) Color change
At certain energy densities (below melting point), materials can undergo chemical changes when exposed to laser radiation of a specific wavelength. The chemical change can be either light or heat induced, e.g. excimer laser induced photo-chemical color change for aircraft cable marking (white to black), and CO2 laser-induced (heat-induced) colour change on PVC (gray to red-brown). The color change is due to changes in chemical composition or in molecular structures.
Pigments are often added into base plastic materials at an appropriate ratio for color change. Following are a few commonly-used pigments.
- inorganic : white TiO2, yellow iron oxide Fe2O3.H2O, black iron oxide Fe3O4; green chromium oxide (Cr2O3 and Cr2O3.2H2O); chrome orange (PbCrO4)x.(PbO)y, cadmium yellow (mixed CdS/ZnS) etc.
- Organic : yellow di-chlorobenzidine derivatives; orange dianisidine derivatives; red toulidine reds etc.
When using pigment, the following factors should be considered:
· selection of pigment with respects to base material (transparency to laser wavelength)
· effects on base material properties
· additional process and cost
· could the color degrade with time?
Figure 5 shows the laser marking through colour change.
Marking through colour change









